Fischer indole synthesis mechanism pdf Nares Inlet
FileFischerIndoleSynthesisMechanism.svg Wikimedia Commons Fischer Indole Synthesis with Organozinc Reagents View Enhanced PDF Access article on Wiley Online Library (HTML view) Download PDF for offline viewing. Logged in as READCUBE_USER. Log out of ReadCube. Abstract. Updated
Fischer Indole Synthesis Chem-Station Int. Ed.
Chemical Science chtf.stuba.sk. Fischer Indole Synthesis in Low Melting Mixtures. Organic Letters 2012, 14 (17) , 4568-4571. DOI: 10.1021/ol302034r. Alex W. Schammel, Grace Chiou, and Neil K. Garg . Interrupted Fischer Indolization Approach toward the Communesin Alkaloids and Perophoramidine., Abstract. Recent data on the mechanism of the rearrangement of arylhydrazones to indoles (the Fischer reaction) are examined. The effect of electronic factors and the acidity of the medium on the rate of the process is discussed..
Fischer Indole Synthesis. The conversion of aryl hydrazones to indoles; requires elevated temperatures and the addition of Brønsted or Lewis acids. Some interesting enhancements have been published recently; for example a milder conversion when N-trifluoroacetyl enehydrazines are used as substrates. . Mechanism of the Fischer Indole Synthesis . Recent Literature. Aryl Hydrazide beyond as SYNTHESIS OF INDOLE FUSED HETEROCYCLIC COMPOUNDS A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY TOLGA KAPTI IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CHEMISTRY JULY 2013 . ii . iii Approval of the thesis: SYNTHESIS OF INDOLE FUSED …
Fischer Indole Synthesis with Organozinc Reagents View Enhanced PDF Access article on Wiley Online Library (HTML view) Download PDF for offline viewing. Logged in as READCUBE_USER. Log out of ReadCube. Abstract. Updated the Fisher indole synthesis[3]. In order to describe the indole synthesis using benzenoid as precursors, we can indentify four bond disconnections, depending on the bond formed during the process (Figure 1.1). Figure 1.1 - Bond disconnections for indoles synthesis from benzenoid precursors 1.1 …
the Fisher indole synthesis[3]. In order to describe the indole synthesis using benzenoid as precursors, we can indentify four bond disconnections, depending on the bond formed during the process (Figure 1.1). Figure 1.1 - Bond disconnections for indoles synthesis from benzenoid precursors 1.1 … 27/08/2014 · General Characteristics Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give substituted indole products. This vintage Fischer indole synthesis is …
A comprehensive survey of the Fischer indole synthesis. Emphasizes three main features of the reaction, its development, the clarification of its mechanism, and the utilization of it and its The Knorr pyrazole synthesis is an organic reaction used to convert a hydrazine or its derivatives and a 1,3-dicarbonyl compound to a pyrazole using an acid catalyst. The mechanism begins with an acid catalyzed imine formation, where in the case of hydrazine derivatives the attack can happen on either carbonyl carbon and result in two possible
The mechanism of the Fischer indole synthesis has been extensively studied, and the accepted mechanism is shown in the chapter. The so‐called abnormal Fischer indolization has been studied by Ishii and Murakani and their coworkers, and the interrupted Fischer indolization has been evaluated theoretically by Houk, Garg, and colleagues. Several This page was last edited on 6 January 2014, at 11:28. Files are available under licenses specified on their description page. All structured data from the file and property namespaces is available under the Creative Commons CC0 License; all unstructured text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply.
20/04/2012 · By David Turnbull • Methods and strategies for the synthesis of five-membered heteroaromatics • Fisher and Bischler indole syntheses Five-membered aromatic heterocycles • Electrophilic substitution reactions of pyrroles, furans and thiophenes • Metallation of five-membered heteroaromatics and use the of directing groups
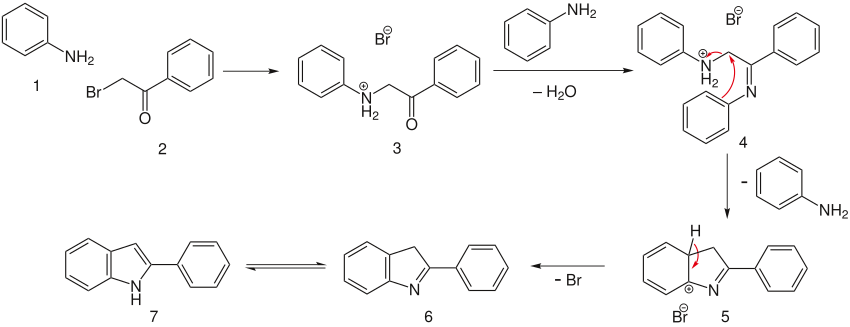
19/02/1949В В· THE mechanism previously proposed1,2 for the Fischer indole reaction has assumed that the intermediate stages involve an o-benzidine-type re-arrangement. This may be illustrated as follows Fischer Indole Synthesis. The conversion of aryl hydrazones to indoles; requires elevated temperatures and the addition of BrГёnsted or Lewis acids. Some interesting enhancements have been published recently; for example a milder conversion when N-trifluoroacetyl enehydrazines are used as substrates. . Mechanism of the Fischer Indole Synthesis . Recent Literature. Aryl Hydrazide beyond as
SYNTHESIS OF INDOLE FUSED HETEROCYCLIC COMPOUNDS A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY TOLGA KAPTI IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CHEMISTRY JULY 2013 . ii . iii Approval of the thesis: SYNTHESIS OF INDOLE FUSED … 27/08/2014 · General Characteristics Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give substituted indole products. This vintage Fischer indole synthesis is …
Castro Indole Synthesis DMF,120ВЎC 17-90% "general and competitive with the Fischer synthesis as a route to 2-substituted indoles" LiCl gave better results regioselective annulation Larock Indole Synthesis I NHR 1 R 2 R 3 c at. Pd(OA)2,h3n-Bu4 ClorLibse N R 1 R 3 R 2 DMF,10ВЎC 16/04/1949В В· Cite this article. PAUSACKER, K. Mechanism of the Fischer Indole Synthesis. Nature 163, 602 (1949) doi:10.1038/163602a0. Download citation. Issue Date. 16 April 1949. DOI
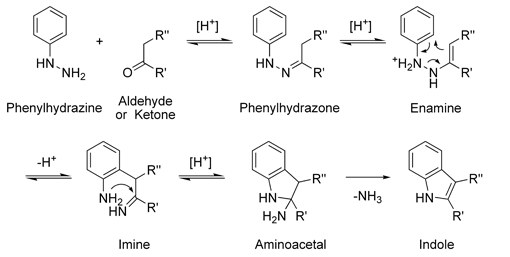
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer.Today antimigraine drugs … A Fischer indole synthesis employing N-Boc arylhydrazines was reported < 04TL1857 >. The Fischer indole synthesis was utilized to prepare pyrrolo[2,3-a]carbazoles 98, truncated analogs of indolo[2,3-a]carbazole < 04JHC349 >. An attempted Fischer cyclization approach to fascaplysin led unexpectedly to a benzo[c]β-carboline product < 04TL1299 >.
Fischer Indole Synthesis SynArchive. Mechanism of the Fischer indole synthesis. Quantum-chemical interpretation of the rearrangement of substituted cyclohexanone arylhydrazones to tetrahydrocarbazoles Quantum-chemical interpretation of the rearrangement of substituted cyclohexanone arylhydrazones to tetrahydrocarbazoles, the Fisher indole synthesis[3]. In order to describe the indole synthesis using benzenoid as precursors, we can indentify four bond disconnections, depending on the bond formed during the process (Figure 1.1). Figure 1.1 - Bond disconnections for indoles synthesis from benzenoid precursors 1.1 ….
Indoles in Organic Synthesis Something Old Something New
Fischer indole synthesis ~ Name-Reaction.com. A Multidisciplinary Investigation into the Design, Synthesis and Evaluation of a Novel Class of Anti-Glioblastoma Drug Fragments. Christopher Sherer, 27/08/2014 · General Characteristics Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give substituted indole products. This vintage Fischer indole synthesis is ….
Indole synthesis organic-chemistry.org
Fischer Indole Synthesis Request PDF ResearchGate. Properties: · The prostaglandins are a large family of lipophilic C-20 hormones. · Isolated in the 1930’s, structures in 1960’s. · Originate from action of COX1 and COX2 on arachidonic acid. Review Peculiarity of methoxy group-substituted phenylhydrazones in Fischer indole synthesis By Yasuoki MURAKAMI*1,†(Communicated by Takao SEKIYA, M.J.A.) Abstract: We found that the Fischer indole synthesis of ethyl pyruvate 2-methoxyphen-.
A: Synthesis of Indoles 1. The Leimgruber-Batcho Synthesis of indole is often used to generate indoles with substituents on the carbocycle. Suggest a mechanism for the final stage of this reaction, which occurs spontaneously: NH 2 N? N R R H 1 Suggest a method of synthesising 1. 2. The first synthesis of indole, discovered in 1883, was the Fischer indole synthesis. This method works well with 11/01/2012В В· In this initial investigation, we focused on 1) the application of the above-mentioned abnormal Fischer indole synthesis, 2) the details of this reaction of phenylhydrazone with other kinds of substituents, 3) the mechanism of the first step of the Fischer indole synthesis, 4) the abnormal reaction in methoxydiphenylhydrazones, and 5) a
Fischer Indole Synthesis in Low Melting Mixtures. Organic Letters 2012, 14 (17) , 4568-4571. DOI: 10.1021/ol302034r. Alex W. Schammel, Grace Chiou, and Neil K. Garg . Interrupted Fischer Indolization Approach toward the Communesin Alkaloids and Perophoramidine. derivative. Several thousand indole derivatives appear annually in chemical literature.3 The Fischer indole synthesis is the most widely used and versatile method for indole synthesis. Reaction Mechanism: The Fischer indole synthesis converts ayrlhydrazones of aldehydes or ketones into indoles in the presence of an acid catalyst.3 Below is the
Abstract. Recent data on the mechanism of the rearrangement of arylhydrazones to indoles (the Fischer reaction) are examined. The effect of electronic factors and the acidity of the medium on the rate of the process is discussed. New data on the mechanism of the Fischer indole synthesis (review). Chemistry of Heterocyclic Compounds 1988, 24 (7) , 709-721. DOI: 10.1007/BF00633160. Graziano Baccolini, Renato …
A: Synthesis of Indoles 1. The Leimgruber-Batcho Synthesis of indole is often used to generate indoles with substituents on the carbocycle. Suggest a mechanism for the final stage of this reaction, which occurs spontaneously: NH 2 N? N R R H 1 Suggest a method of synthesising 1. 2. The first synthesis of indole, discovered in 1883, was the Fischer indole synthesis. This method works well with Mechanism of the Fischer indole synthesis. Quantum-chemical interpretation of the rearrangement of substituted cyclohexanone arylhydrazones to tetrahydrocarbazoles Quantum-chemical interpretation of the rearrangement of substituted cyclohexanone arylhydrazones to tetrahydrocarbazoles
The Knorr pyrazole synthesis is an organic reaction used to convert a hydrazine or its derivatives and a 1,3-dicarbonyl compound to a pyrazole using an acid catalyst. The mechanism begins with an acid catalyzed imine formation, where in the case of hydrazine derivatives the attack can happen on either carbonyl carbon and result in two possible The synthesis of indole derivatives by the treatment of aryl hydrazones coupled from aromatic hydrazines and ketones or aldehydes with either a mineral or Lewis acid is generally known as the Fischer indole synthesis. Although the mechanism of this reaction has been extensively studied, the one formulated by Robinson and Robinson is now
Abstract. Recent data on the mechanism of the rearrangement of arylhydrazones to indoles (the Fischer reaction) are examined. The effect of electronic factors and the acidity of the medium on the rate of the process is discussed. 20/04/2011 · Indole derivatives continue to receive substantial interest due to their wide range of biological activity. 1–5 The Fischer indole synthesis 6 remains among the most widely used approaches to indoles, with more than 700 reports over the last 15 years. 5 Despite the extensive application of the Fischer indole sequence, certain substitution patterns cause the reaction to fail.
The Knorr pyrazole synthesis is an organic reaction used to convert a hydrazine or its derivatives and a 1,3-dicarbonyl compound to a pyrazole using an acid catalyst. The mechanism begins with an acid catalyzed imine formation, where in the case of hydrazine derivatives the attack can happen on either carbonyl carbon and result in two possible • Methods and strategies for the synthesis of five-membered heteroaromatics • Fisher and Bischler indole syntheses Five-membered aromatic heterocycles • Electrophilic substitution reactions of pyrroles, furans and thiophenes • Metallation of five-membered heteroaromatics and use the of directing groups
This chapter focuses on recent applications in drug development, materials discovery, and natural-product synthesis. The mechanism of the Fischer indole synthesis has been extensively studied, and • Methods and strategies for the synthesis of five-membered heteroaromatics • Fisher and Bischler indole syntheses Five-membered aromatic heterocycles • Electrophilic substitution reactions of pyrroles, furans and thiophenes • Metallation of five-membered heteroaromatics and use the of directing groups
the Fisher indole synthesis[3]. In order to describe the indole synthesis using benzenoid as precursors, we can indentify four bond disconnections, depending on the bond formed during the process (Figure 1.1). Figure 1.1 - Bond disconnections for indoles synthesis from benzenoid precursors 1.1 … Abstract. Recent data on the mechanism of the rearrangement of arylhydrazones to indoles (the Fischer reaction) are examined. The effect of electronic factors and the acidity of the medium on the rate of the process is discussed.
This page was last edited on 6 January 2014, at 11:28. Files are available under licenses specified on their description page. All structured data from the file and property namespaces is available under the Creative Commons CC0 License; all unstructured text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply. • Methods and strategies for the synthesis of five-membered heteroaromatics • Fisher and Bischler indole syntheses Five-membered aromatic heterocycles • Electrophilic substitution reactions of pyrroles, furans and thiophenes • Metallation of five-membered heteroaromatics and use the of directing groups
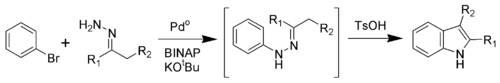
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer.Today antimigraine drugs … Castro Indole Synthesis DMF,120¡C 17-90% "general and competitive with the Fischer synthesis as a route to 2-substituted indoles" LiCl gave better results regioselective annulation Larock Indole Synthesis I NHR 1 R 2 R 3 c at. Pd(OA)2,h3n-Bu4 ClorLibse N R 1 R 3 R 2 DMF,10¡C
Fischer Indole Synthesis YouTube
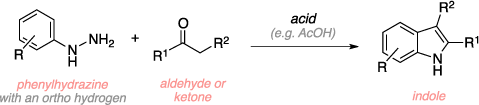
Fischer Indole Synthesis organic-chemistry.org. One of the oldest and most useful reactions in organic chemistry is the Fischer indole synthesis (FIS). It is known to have a wide variety of applications including the synthesis of indole rings, often present as the framework in the total synthesis of natural products, particularly those found in the realm of alka 2017 Review articles, 12/09/2016В В· Fischer indole synthesis.
Fischer Indole Synthesis YouTube
Fischer Indole Synthesis an overview ScienceDirect Topics. This chapter focuses on recent applications in drug development, materials discovery, and natural-product synthesis. The mechanism of the Fischer indole synthesis has been extensively studied, and, THE mechanism previously proposed 1,2 for the Fischer indole reaction has assumed that the intermediate stages involve an o-benzidine-type re-arrangement. This may be illustrated as follows, considering, as an example, the phenyl hydrazone of cyclohex-anone :.
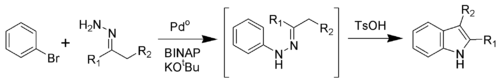
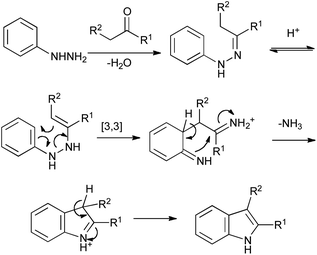
The Fischer indole synthesis is an organic reaction used to convert a phenyl hydrazine and an aldehyde or ketone to an indole using an acid catalyst. The mechanism begins with formation of a phenylhydrazone through the acid catalyzed reaction of the hydrazine with the carbonyl. The phenylhydrazone then rearranges to the enamine and gets Photoredox Fischer Indole Synthesis. Photoredox Fischer Indole Synthesis: A. Kaga, T. Fukushima, J. Shimokawa, M. Kitamura Synthesis 2019, DOI: 10.1055/s-0037-1611535. The Fischer indole synthesis is a classic process in organic chemistry.
Fischer Indole Synthesis with Organozinc Reagents View Enhanced PDF Access article on Wiley Online Library (HTML view) Download PDF for offline viewing. Logged in as READCUBE_USER. Log out of ReadCube. Abstract. Updated applied to the synthesis of eletriptan (Scheme 7), and the reduc-tion step has also been demonstrated in ow.11 Scheme 3 Classical routes to indoles from ortho-substituted anilines. Scheme 4 The Fischer indole synthesis. Scheme 5 Garg’s synthesis of aspidophylline using a Fischer indole approach.
This page was last edited on 6 January 2014, at 11:28. Files are available under licenses specified on their description page. All structured data from the file and property namespaces is available under the Creative Commons CC0 License; all unstructured text is available under the Creative Commons Attribution-ShareAlike License; additional terms may apply. applied to the synthesis of eletriptan (Scheme 7), and the reduc-tion step has also been demonstrated in ow.11 Scheme 3 Classical routes to indoles from ortho-substituted anilines. Scheme 4 The Fischer indole synthesis. Scheme 5 Garg’s synthesis of aspidophylline using a Fischer indole approach.
Properties: · The prostaglandins are a large family of lipophilic C-20 hormones. · Isolated in the 1930’s, structures in 1960’s. · Originate from action of COX1 and COX2 on arachidonic acid. The Knorr pyrazole synthesis is an organic reaction used to convert a hydrazine or its derivatives and a 1,3-dicarbonyl compound to a pyrazole using an acid catalyst. The mechanism begins with an acid catalyzed imine formation, where in the case of hydrazine derivatives the attack can happen on either carbonyl carbon and result in two possible
27/08/2014 · General Characteristics Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give substituted indole products. This vintage Fischer indole synthesis is … applied to the synthesis of eletriptan (Scheme 7), and the reduc-tion step has also been demonstrated in ow.11 Scheme 3 Classical routes to indoles from ortho-substituted anilines. Scheme 4 The Fischer indole synthesis. Scheme 5 Garg’s synthesis of aspidophylline using a Fischer indole approach.
Fischer indole synthesis The classical Fischer indole synthesis involves cyclization of arylhydrazones of carbonyl compounds under the action of acidic agents (Scheme 1).26 Scheme 1 The modern modifications of the method include the change in the reaction conditions, application of new catalysts and approaches to the synthesis of starting aryl- hydrazines and hydrazones and the use of unusual A Multidisciplinary Investigation into the Design, Synthesis and Evaluation of a Novel Class of Anti-Glioblastoma Drug Fragments. Christopher Sherer
Fischer Indole Synthesis in Low Melting Mixtures. Organic Letters 2012, 14 (17) , 4568-4571. DOI: 10.1021/ol302034r. Alex W. Schammel, Grace Chiou, and Neil K. Garg . Interrupted Fischer Indolization Approach toward the Communesin Alkaloids and Perophoramidine. One of the oldest and most useful reactions in organic chemistry is the Fischer indole synthesis (FIS). It is known to have a wide variety of applications including the synthesis of indole rings, often present as the framework in the total synthesis of natural products, particularly those found in the realm of alka 2017 Review articles
Media in category "Fischer indole synthesis" The following 26 files are in this category, out of 26 total. One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis, developed in 1883 by Emil Fischer. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions.
1. Batcho-Leimgruber Indole Synthesis 2. Reissert Indole Synthesis 3. Hegedus Indole Synthesis 4. Fukuyama Indole Synthesis 5. Sugasawa Indole Synthesis 6. Bischler Indole Synthesis 7. Gassman Indole Synthesis 8. Fischer Indole Synthesis 9. Japp-Klingemann Indole Synthesis 10. Buchwald Indole Synthesis 11. Bucherer Carbazole Synthesis 12. Japp 12/09/2016В В· Fischer indole synthesis
THE mechanism previously proposed1,2 for the Fischer indole reaction has assumed that the intermediate stages involve an o-benzidine-type re-arrangement. This may be illustrated as follows THE mechanism previously proposed 1,2 for the Fischer indole reaction has assumed that the intermediate stages involve an o-benzidine-type re-arrangement. This may be illustrated as follows, considering, as an example, the phenyl hydrazone of cyclohex-anone :
Mechanism of the Fischer Indole Synthesis Nature. Properties: · The prostaglandins are a large family of lipophilic C-20 hormones. · Isolated in the 1930’s, structures in 1960’s. · Originate from action of COX1 and COX2 on arachidonic acid., Review Peculiarity of methoxy group-substituted phenylhydrazones in Fischer indole synthesis By Yasuoki MURAKAMI*1,†(Communicated by Takao SEKIYA, M.J.A.) Abstract: We found that the Fischer indole synthesis of ethyl pyruvate 2-methoxyphen-.
Indole synthesis a review and proposed classification
Richter Indole and Pyrrole Synthesis 9/1/04 Group Meeting. Fischer Indole Synthesis in Low Melting Mixtures. Organic Letters 2012, 14 (17) , 4568-4571. DOI: 10.1021/ol302034r. Alex W. Schammel, Grace Chiou, and Neil K. Garg . Interrupted Fischer Indolization Approach toward the Communesin Alkaloids and Perophoramidine., Abstract. Recent data on the mechanism of the rearrangement of arylhydrazones to indoles (the Fischer reaction) are examined. The effect of electronic factors and the acidity of the medium on the rate of the process is discussed..
Peculiarity of methoxy group-substituted phenylhydrazones

Indole synthesis a review and proposed classification. 27/08/2014 · General Characteristics Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give substituted indole products. This vintage Fischer indole synthesis is … This chapter focuses on recent applications in drug development, materials discovery, and natural-product synthesis. The mechanism of the Fischer indole synthesis has been extensively studied, and.
23/09/2011 · The division of strategies is strictly operational. Thus, the Fischer indole synthesis is classified as Type 1, Ar–H to C2, since that is the way it is carried out, even though the last bond formed, as the reaction proceeds, is in fact N to C1. A comprehensive survey of the Fischer indole synthesis. Emphasizes three main features of the reaction, its development, the clarification of its mechanism, and the utilization of it and its
16/04/1949В В· Cite this article. PAUSACKER, K. Mechanism of the Fischer Indole Synthesis. Nature 163, 602 (1949) doi:10.1038/163602a0. Download citation. Issue Date. 16 April 1949. DOI 11/01/2012В В· In this initial investigation, we focused on 1) the application of the above-mentioned abnormal Fischer indole synthesis, 2) the details of this reaction of phenylhydrazone with other kinds of substituents, 3) the mechanism of the first step of the Fischer indole synthesis, 4) the abnormal reaction in methoxydiphenylhydrazones, and 5) a
The Synthesis of 2- and 3-Substituted Indoles Tlabo Caiphus Leboho A dissertation submitted to the Faculty of Science, University of the Witwatersrand, Johannesburg, in fulfilment of the requirement for the degree of Master of Science . January 2008 . Declaration i Declaration I declare that the work presented in this dissertation was carried out exclusively by myself under the supervision of 1. Batcho-Leimgruber Indole Synthesis 2. Reissert Indole Synthesis 3. Hegedus Indole Synthesis 4. Fukuyama Indole Synthesis 5. Sugasawa Indole Synthesis 6. Bischler Indole Synthesis 7. Gassman Indole Synthesis 8. Fischer Indole Synthesis 9. Japp-Klingemann Indole Synthesis 10. Buchwald Indole Synthesis 11. Bucherer Carbazole Synthesis 12. Japp
Abstract. Recent data on the mechanism of the rearrangement of arylhydrazones to indoles (the Fischer reaction) are examined. The effect of electronic factors and the acidity of the medium on the rate of the process is discussed. Properties: · The prostaglandins are a large family of lipophilic C-20 hormones. · Isolated in the 1930’s, structures in 1960’s. · Originate from action of COX1 and COX2 on arachidonic acid.
THE mechanism previously proposed1,2 for the Fischer indole reaction has assumed that the intermediate stages involve an o-benzidine-type re-arrangement. This may be illustrated as follows 11/01/2012В В· In this initial investigation, we focused on 1) the application of the above-mentioned abnormal Fischer indole synthesis, 2) the details of this reaction of phenylhydrazone with other kinds of substituents, 3) the mechanism of the first step of the Fischer indole synthesis, 4) the abnormal reaction in methoxydiphenylhydrazones, and 5) a
Review Peculiarity of methoxy group-substituted phenylhydrazones in Fischer indole synthesis By Yasuoki MURAKAMI*1,†(Communicated by Takao SEKIYA, M.J.A.) Abstract: We found that the Fischer indole synthesis of ethyl pyruvate 2-methoxyphen- Fischer Indole Synthesis. The conversion of aryl hydrazones to indoles; requires elevated temperatures and the addition of Brønsted or Lewis acids. Some interesting enhancements have been published recently; for example a milder conversion when N-trifluoroacetyl enehydrazines are used as substrates. . Mechanism of the Fischer Indole Synthesis . Recent Literature. Aryl Hydrazide beyond as
20/04/2011 · Indole derivatives continue to receive substantial interest due to their wide range of biological activity. 1–5 The Fischer indole synthesis 6 remains among the most widely used approaches to indoles, with more than 700 reports over the last 15 years. 5 Despite the extensive application of the Fischer indole sequence, certain substitution patterns cause the reaction to fail. Photoredox Fischer Indole Synthesis. Photoredox Fischer Indole Synthesis: A. Kaga, T. Fukushima, J. Shimokawa, M. Kitamura Synthesis 2019, DOI: 10.1055/s-0037-1611535. The Fischer indole synthesis is a classic process in organic chemistry.
THE mechanism previously proposed1,2 for the Fischer indole reaction has assumed that the intermediate stages involve an o-benzidine-type re-arrangement. This may be illustrated as follows Castro Indole Synthesis DMF,120ВЎC 17-90% "general and competitive with the Fischer synthesis as a route to 2-substituted indoles" LiCl gave better results regioselective annulation Larock Indole Synthesis I NHR 1 R 2 R 3 c at. Pd(OA)2,h3n-Bu4 ClorLibse N R 1 R 3 R 2 DMF,10ВЎC
Fischer indole synthesis The classical Fischer indole synthesis involves cyclization of arylhydrazones of carbonyl compounds under the action of acidic agents (Scheme 1).26 Scheme 1 The modern modifications of the method include the change in the reaction conditions, application of new catalysts and approaches to the synthesis of starting aryl- hydrazines and hydrazones and the use of unusual One of the oldest and most useful reactions in organic chemistry is the Fischer indole synthesis (FIS). It is known to have a wide variety of applications including the synthesis of indole rings, often present as the framework in the total synthesis of natural products, particularly those found in the realm of alka 2017 Review articles
• Methods and strategies for the synthesis of five-membered heteroaromatics • Fisher and Bischler indole syntheses Five-membered aromatic heterocycles • Electrophilic substitution reactions of pyrroles, furans and thiophenes • Metallation of five-membered heteroaromatics and use the of directing groups 1. Batcho-Leimgruber Indole Synthesis 2. Reissert Indole Synthesis 3. Hegedus Indole Synthesis 4. Fukuyama Indole Synthesis 5. Sugasawa Indole Synthesis 6. Bischler Indole Synthesis 7. Gassman Indole Synthesis 8. Fischer Indole Synthesis 9. Japp-Klingemann Indole Synthesis 10. Buchwald Indole Synthesis 11. Bucherer Carbazole Synthesis 12. Japp
Properties: · The prostaglandins are a large family of lipophilic C-20 hormones. · Isolated in the 1930’s, structures in 1960’s. · Originate from action of COX1 and COX2 on arachidonic acid. Media in category "Fischer indole synthesis" The following 26 files are in this category, out of 26 total.